Background
Current guidelines recommend upfront autologous stem cell transplantation (ASCT) as part of first line treatment of newly diagnosed multiple myeloma (NDMM). These recommendations are based on randomized controlled trials conducted in patients aged 18-65 years. While guidelines recommend the same approach for selected older patients, the use of ASCT in these patients is not well documented. The median age of NDMM is 71 years in recent population-based studies, and the prevalence of MM is increasing. Recent data from the European Society for Blood and Marrow Transplantation centers (EBMT), show that an increasing number of patients above the age of 65 receive ASCT. However, it is unknown whether this reflects an increasing number of transplant eligible NDMM, or a true change in clinical treatment practice.
Methods
We conducted a population-based, nationwide study from 6 countries in the Nordic and Baltic regions. Patients diagnosed with MM between January 1st 2008 and December 31st 2020 in Sweden, Denmark, Norway, Lithuania, Estonia and Iceland were included in the study. Data were collected from the Swedish and Danish Myeloma Registries, from chart review in the other 4 countries, and supplemented with data from the Norwegian and Icelandic nationwide Cancer Registries. Aggregated data on ASCT use and survival from the participating countries were combined for analysis. Patients were divided into 3 age groups: 18-65, 66-70 and 71-75 years, and compared with the total population of patients diagnosed with MM. Changes in the rate of ASCT over time were assessed by log-binomial regression. Because registration of date of death is virtually complete in these countries, 1-year, 3-year, and 5-year overall survival were estimated using empirical survival at these time points with 95% confidence intervals (CI) calculated using bootstrapping with 1000 repeats. We chose the combined endpoint of very good partial response (VGPR) or better and surviving one year after ASCT, as a surrogate for a successful outcome of first line treatment. A similar statistical method was used for this endpoint as for survival.
Results
In total, 5,644 patients were treated with ASCT (18-65 (n=4,596), 66-70 (n=970) and 71-75 years (n=78)). Baseline characteristics of patients were similar for all the countries, with a mean age at diagnosis of 58.7 years. There were differences between the countries regarding induction regimens. In total 55% received PI-based induction, while 29% received both proteasome inhibitor (PI) and immunomodulatory agent.
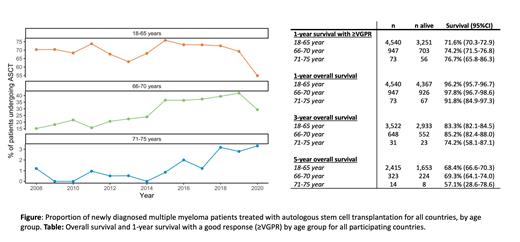
The mean proportion of patients in the age group 18-65 treated with upfront ASCT was 69% and did not change over the study period (relative risk (RR) of ASCT: 0.95; 95% CI: 0.89-1.01). In contrast, the mean proportion of NDMM patients in the age group 66-70 treated with upfront ASCT was 28%, and increased more than 2.5-fold during the study period (RR of ASCT: 2.64; 95% CI: 2.14-3.27). Only 1.2% of patients 71-75 years were treated with ASCT, with an increasing trend in the most recent years (Figure).
The proportion of patients achieving VGPR or better and surviving one year after ASCT was similar in all three age groups: 18-65 years (71.6% (70.3-72.9)), 66-70 years (74.2% (71.5-76.8)), 71-75 years (76.7% (65.8-86.3)) (Table). The 3-year and 5-year survival in patients >65 years treated with ASCT was stable throughout the study period.
Conclusion
In conclusion, we find that the proportion of patients with NDMM aged 66-70 treated with ASCT is increasing. This is due to a change in clinical practice. The efficacy of ASCT in patients aged 18-65 and 66-70 was similar. Our real-world population-based data supports the clinical practice of an ambitious approach, including ASCT, in the treatment of selected NDMM patients up to the age of 70 and perhaps beyond.
Disclosures
Nordberg Nørgaard:Janssen: Honoraria. Peceliunas:Amgen: Consultancy, Other: Travel expenses; Johnson & Johnson: Consultancy, Other: Travel expenses. Schjesvold:Daiichi Sankyo: Other: Honoraria for lectures and educational material; Celgene: Consultancy, Other: Honoraria for lectures and educational material, Research Funding; Takeda: Consultancy, Other: Honoraria for lectures and educational material; Janssen-Cilag: Consultancy, Other: Honoraria for lectures and educational material, Research Funding; Oncopeptides: Consultancy, Other: Honoraria for lectures and educational material, Research Funding; Sanofi: Consultancy, Other: Honoraria for lectures and educational material, Research Funding; Bristol Myers Squibb: Consultancy, Other: Honoraria for lectures and educational material; Targovax: Research Funding; Amgen: Other: Honoraria for lectures and educational material; Novartis: Other: Honoraria for lectures and educational material; Pfizer: Other: Honoraria for lectures and educational material; Skylite DX: Other: Honoraria for lectures and educational material; GlaxoSmithKline: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Other: Honoraria for lectures and educational material; Schain: Other: Honoraria for lectures and educational material. Szabo:BMS: Research Funding; Takeda: Consultancy, Research Funding; Janssen: Consultancy; Sanofi: Consultancy. Blimark:Sanofi: Honoraria; Takeda: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; BMS: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal